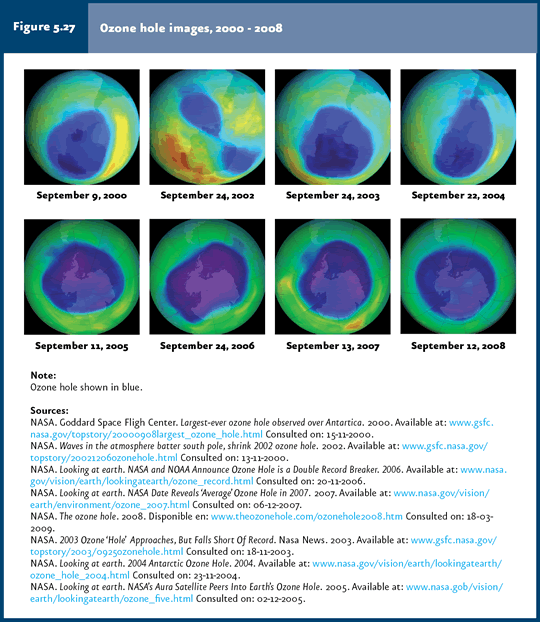
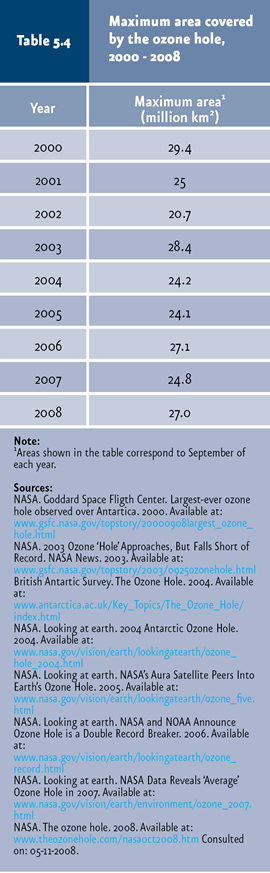
In 2008, the ozone hole stretched over an area of 27 million square kilometers, which is slightly larger than North America. |
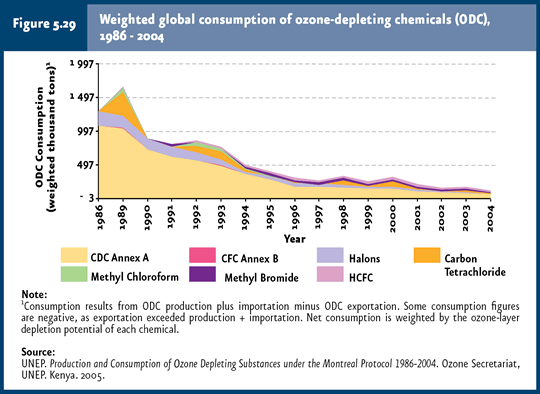
Global ODS consumption underwent a 90% reduction during 1986-2004. |
The national ODS consumption decreased 95% in 1989-2007. |
Mexico has complied with the reduction goals for the consumption of several ODS, including CFC, TET and MCF. |
| CHAPTER 5. ATMOSPHERE |
The thinning of the ozone layer represents another of the key global environmental issues, since this layer filters UV rays emitted by the Sun. UV rays belong to three types: UV-A, UV-B and UV-C. UV-A is the least harmful, entering in lesser amounts to the Earth surface. UV-C rays are the most harmful, due to their high energy content, but the ozone layer prevents them from going through the atmosphere. Last, UV-B rays, also considerably harmful, are mostly filtered by the ozone layer, although a slight portion reaches the Earth surface, with the potential to damage cells and tissues of living organisms. The ozone layer has been thinning as a result of the effect of a number of agents, generically known as ozone depletion substances (ODS). These contain chlorine, bromine and fluorine atoms in their structure, derived mostly from human activities. The best known ODS are chlorofluorocarbons (CFC), but also hydrochlorofluorocarbons (HCFC), halons, methyl bromide (MBR), carbon tetrachloride (TET) and methyl chloroform are included in this category. ODS are commonly used in cooling and air-conditioning systems, polyurethane rigid foam, solvents, pesticides, aerosols and fire extinguishers, among others. When these compounds are released to the atmosphere, ODS reach the stratosphere, where they participate in a series of reactions leading to the release of chlorine and bromine atoms which may destroy ozone; one chlorine or bromine molecule may destroy one thousand ozone molecules (WMO & UNEP, 2003). Although ODS emissions are released throughout the world and the ozone layer thinning is a global phenomenon, atmospheric circulation transports most ODS towards the poles. The climatic conditions that prevail in the southern Pole favor reactions which convert ODS in reactive gases which destroy ozone. This destruction occurs on the ice particle surface of stratospheric clouds formed on the Antarctica in the winter. These reactions release active chlorine and bromine moieties which accumulate in polar clouds. During the spring, as temperature rises, clouds disgregate and release active chlorine and bromine, which rapidly destroy ozone. For this reason, although this is a global issue, its effects are less important near the equator and increase with latitude towards the poles, particularly in the case of the southern Pole (PNUMA, 2002, 2003; WMO & UNEP, 2003). The thinning of the ozone layer in Antarctica has produced the so-called “ozone hole14”, observed for the first time in the early 1980s and displaying its largest recorded size in 2000, when it stretched over nearly 29.4 million km2 (Figure 5.27 and Table 5.4). In 2008, the maximum size was 27 million km2, an area slightly larger than North America (NASA, 2008).
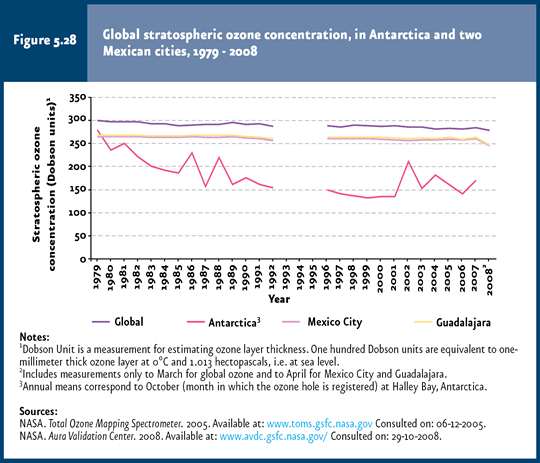
Figure 5.28 shows global stratospheric ozone concentrations for Antarctica, Mexico City and Guadalajara between 1979 and 2008. The low concentration recorded since the early 1980s (October) in Antarctica is evident compared to the global concentration (IB 1.3-3). A declining trend in ozone concentration has been maintained in that region ever since, with levels below the global concentrations. By contrast, both the global concentrations and the one in the two Mexican cities shown as reference evidence no significant changes in their respective concentration curves, stressing the fact that, despite of this phenomenon being a global issue, its consequences may vary regionally.
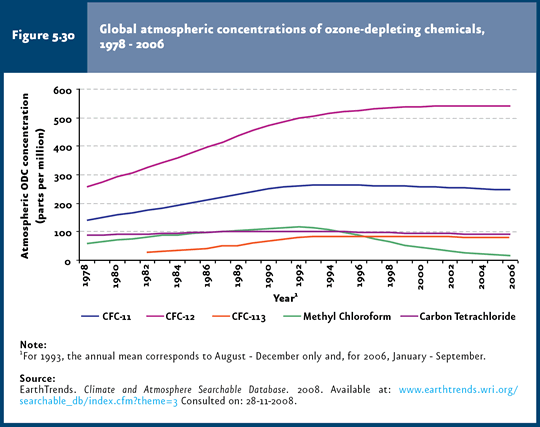
ODS consumption and concentration Given that the impact derived from ODS depends on their ozone depletion potential15 (WMO & UNEP, 2003), this chapter deals with consumption, both globally and in the country, weighed according to such potential. It is worth noting that weighed ODS consumption considers the life cycle of these substances in an integrated manner (production, importation and exportation), as well as their specific capacity to destroy ozone. Despite global ODS consumption markedly declined in the early 1990s (90% reduction in the period 1986-2004), this has yet to be reflected in atmospheric concentrations (Figures 5.29 and 5.30;IB 1.3-1 and 1.3-4). This is due to that ODS have been accumulating steadily in the stratosphere, so that the increase in concentration has stopped and their levels have remained virtually unchanged since the early 1990s (WMO & UNEP, 2003).
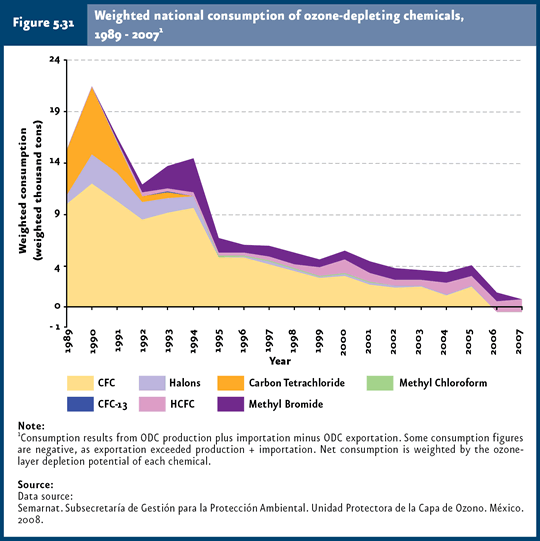
The most optimistic scenarios predict that the ozone layer may start recovering within the next 10 to 20 years, and its full recovery would be reached after the first half of the 21st century (PNUMA, 2002). This uncertainty partly derives a number of studies which affirm that longer historical series are required, along with a better understanding of atmospheric processes and their effects on ozone, before being able to predict when the ozone layer will recover. Besides the atmospheric ODS concentration, there are additional factors that influence ozone destruction, such as stratospheric temperature, solar activity, atmospheric concentration of gases including methane, water vapor and nitrous oxide (Weatherhead & Andersen, 2006; refer to Box Are climate change and ozone-layer thinning related?). In Mexico, total weighted ODS consumption decreased 95% in 2007 (764 tons were consumed) compared with 1989 figures (15 thousand tons when the Montreal Protocol16 came into force; Figure 5.31; IB 1.3-2; IC9). This decrease is mainly due to the elimination of CFC use in compliance with Mexico’s goals to protect the ozone layer.
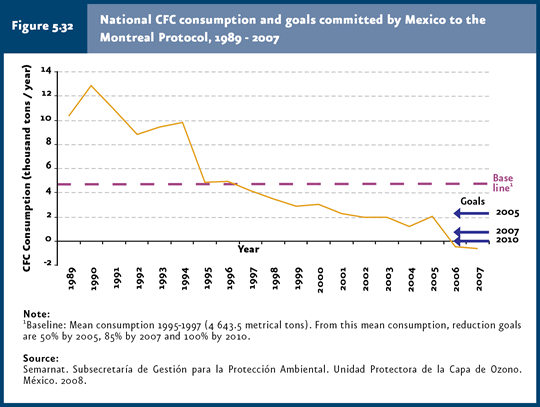
Protection of the ozone layer Concerns by the scientific community and governments of several countries because of the stratospheric ozone loss led to the adoption of the Vienna Convention for the Protection of the Ozone Layer (1985) and the Montreal Protocol on Substances that Deplete the Ozone Layer (1987), which set forth commitments to reduce ODS consumption and production (PNUMA, 2003). Mexico signed both treaties, and adopted the London, Copenhagen and, most recently, Montreal (2006) and Beijing (2007) amendments. By 1995, the production of most ozone-depleting substances included in the Montreal Protocol had stopped in industrialized countries. In the case of developing countries, the Protocol specified a 10-year deadline for their elimination, and, additionally, financial support was offered to allow these countries to face the costs associated with ODS elimination. Besides decreasing ODS consumption, Mexico has acted in advance to international controls, since it reached the committed goals prior to the deadline. The strategy adopted by Mexico is based on the following measures: 1) control of ODS consumption and production, 2) promotion and advisory on the use of alternate substances that minimize impacts on the ozone layer, 3) introduction of clean technologies that use substances other than ODS, and 4) training to users on ozone-layer conservation measures. This strategy has been implemented considering the reduction schedule committed by countries to the Montreal Protocol. Among the goals committed by Mexico, it is worth mentioning that, as regards CFCs, carbon tetrachloride (TET) and methyl chloroform (MCF), Mexico complied with the goals previously set. For example, a negative CFC consumption was reported in 2007, since the production of these substances was discontinued and exports exceeded imports (Figure 5.32). For further information on ODS production, importation and exportation in Mexico, please refer to Boxes: D3_AIRE03_01, D3_AIRE03_02, D3_AIRE03_03 and D3_AIRE03_04.
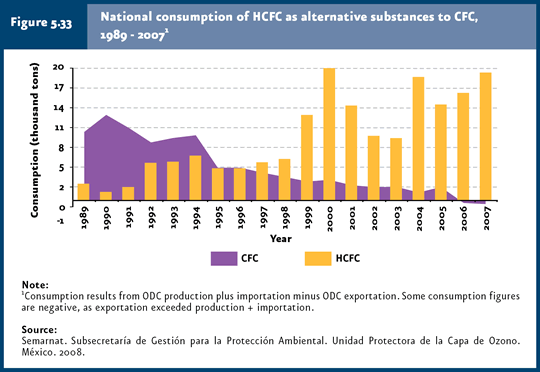
As a result of the encouragement for using alternate substances, some have been used which are less harmful to the ozone layer. For example, HCFCs have replaced CFCs, given the former’s lower ozone-depletion potential. In Mexico, HCFCs consumed have depletion potentials between 0.04 and 0.07, while CFCs range from 0.6 to 1.0. Figure 5.33 shows the outcome of CFC replacement, that is, while CFCs used in cooling and air-conditioning systems has dropped, HCFC consumption is on the rise (IB 1.3-5).
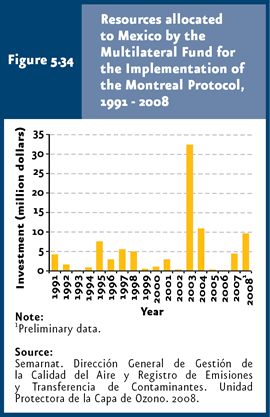
To note, even for HCFCs there are consumption reduction goals. This replacement, as well as the generalized decrease in ODS consumption, has resulted from the support to projects investing in clean technologies in sectors that use these substances, such as those dealing with cooling systems, air conditioning, solvents, polyurethane foams and fumigation, among others. To assist developing countries in complying with control measures adopted by the Montreal Protocol, the Multilateral Fund for the Implementation of the Montreal Protocol was created at an international level (PNUMA, 2003; UNEP, 2001, 2003). Fund resources are allocated to fostering the introduction of clean technologies and provide training to ODS users. In 1991-2008, this Fund dedicated some 2 thousand 400 million USD worldwide to support developing countries in project implementation; a little over 91.5 million USD were granted to Mexico (Figure 5.34).
At a country level and aiming at implementing the measures required for complying with Mexico’s commitments to the Montreal Protocol, the Unit for the Protection of the Ozone Layer, coordinated by Semarnat, was created more than ten years ago (Semarnat, 2007). The achievements reached include the elimination of CFC consumption as a result of the execution of more than 100 projects for the replacement of these substances in household and commercial fridges, air conditioners, aerosols, solvents and polyurethane foams. As part of the CFC elimination strategy, the CFC production plant was closed in 2005, an action through which Mexico was four years ahead of the goals set forth by the Montreal Protocol. By doing this, besides discontinuing production in Mexico, a 12% reduction of the world production and a 60% reduction of that in Latin America were achieved. Separately, the Information and Monitoring System on the Importation, Exportation and Production of Ozone-Layer Depleting Substances (SISSAO in Spanish) has been developed to oversee the importation and exportation of these substances and build statistical records. Additionally, projects are being executed to provide technical assistance and training to methyl bromide users – a substance used as pesticide in soil and grain / flour storage systems – aimed at reducing consumption.
Notes 15International treaty which sets forth the reduction commitments on ODS production and consumption aimed at protecting the ozone layer. 16International treaty which sets forth the reduction commitments on ODS production and consumption aimed at protecting the ozone layer.
|