The rain, deposition or acid precipitation is made from the chemical reaction of its precursors, sulphur dioxide (SO2) and nitrogen oxides (NOx), with the atmospheric humidity. The sulphur and nitrogen acids that are formed are deposited in constructions and monuments, vegetation, soil and water bodies through gases or particles (dry deposition) or rain, snow and fog (wet deposition).
In order to detect the presence of acid rain in a specific region the value of pH1 of the water rain is used as a reference, such value is 5.6 (SMAGDF et al., 2011). The acid rain precursors come from natural sources, such as the forestal fires, the volcanic emissions and the decomposing organic matter, or from anthropogenic sources related to the agriculture and the fossil fuels burning in the industry, the power generation and the transportation sector (EPA, 2011; SMAGDF et al., 2011). The effects of the acid deposition (dry and wet) depend on several factors, such as the acidity level of the water, the chemical composition and the buffering capacity of the material where it falls, as well as the susceptibility of the vegetation and the organisms exposed to it (INE, 2007).
Effects of acid rain
The acid rain may affect, practically, all the ecosystems. It reaches the water bodies directly for the pluvial events or by the runoffs of the nearby areas (EPA, 2011). The runoffs may also drag toxic elements such as the aluminum, which makes the problem of water acidification worse because it directly affects the organisms (Xu and Ji, 2001). The acid rain may cause the acidification of lakes and streams with a low buffering capacity; the lakes which have a pH between 6 and 8 may mitigate in a better way the acid effect of the rain; while the ones that are naturally acid, present a minor buffering capacity (EPA, 2011).
The acidification of the water bodies has several consequences in the ecosystems and, particularly in the food webs. For instance, it has been observed the decrease of the aquatic invertebrate populations as well as the weight and height of the fish (EPA, 2011), what also has an impact in the reproductive success and the abundance of birds which feed from them (Graveland, 1998).
In the terrestrial ecosystems the rain acidity causes the leaching of the soil nutrients before the plants may use them, causes damages and photosynthetic alterations in the leaves and changes in the physic-chemical properties of the soil (Calva et al., 1991; Saavedra-Romero et al., 2003). At a world level, it has been estimated that between 7 and 17% of the terrestrial ecosystems are at a critical risk of acidification (Bouwman et al., 2002). The acid precipitation also damages the agricultural crops as it damages the leaves and reduces the quality of the soil (SMAGDF et al., 2011).
There have been several studies which have been carried out in Mexico to evaluate the effect of the acid rain on the ecosystems, particularly in the forests that surround the Valley of Mexico Metropolitan Zone (VMMZ). In Chapa de Mota and San Luis Ayucan at northeast the Valley of Mexico there have very acid records in the rain (Velasco-Saldaña et al., 2002). In the “Desierto de los Leones” National Park, in the forests of Abies religiosa there have been records of pH values between 5.11 and 6.64 in the rain (Saavedra-Romero et al., 2003). This acidity is related to different types of vegetation damage such as the loss of leaves and branches, leaf necrosis, chlorosis, debarking and nutritional deficiency (Saavedra-Romero et al., 2003).
In addition to their effects over the forest ecosystem, the acid rain also damages the limestone rock of buildings and historical monuments. For instance, Bravo et al. (2006) documented the damages in the archeological area El Tajín, in Veracruz, where there have been recorded values of pH below 5.62 El Tajín is surrounded by potential sources of acid rain precursors with a high content of sulphur (electric plants and refineries), which are transported by the wind currents that usually go through the southeast of the Gulf of Mexico.
Acid rain monitoring in the VMMZ
At a national level there is not a program of specific monitoring for the acid rain; however, in the decade of 1980s the first researches about their presence, characterization and effects on the VMMZ were made. In 1987, the systematic monitoring was implemented, but it was until 2001 that the Atmospheric Deposit Network (REDDA in Spanish) was consolidated, and later it was integrated into the Atmosphere Monitoring System (SIMAT in Spanish). Until 2006, the REDDA was made up of 16 monitoring stations scattered in the urban, rural and ecological conservation areas of the whole VMMZ. In these stations, the pH and the concentration of ions present in the wet deposition are recorded (Muñoz et al., 2008).
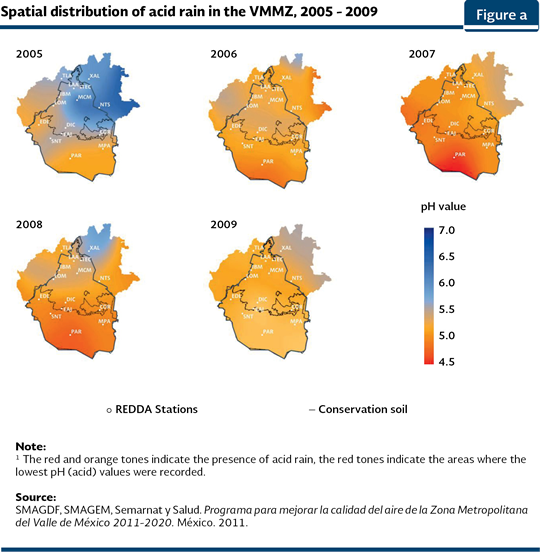
Although, in the VMMZ, SO2 and NOx emissions are higher in the central, northeast and northwest regions, which are related to the weight of traffic, the winds have a north-southeast direction, what allows the pollutants to be moved towards the south of Mexico City, where they accumulate leading to acid precipitations. The historical records in the VMMZ show that in 1989 it was recorded the most acid value (3.4; INE, 2007). Figure a shows the behavior of the acid rain in the last years and it is evident that the intensity of the phenomenon varies among years, the affected surface has increased and the most affected area is the one in the south-southwest of the city.
Note:
1 It is a measure that determines the acidity or alkalinity of given solution. The scale goes from 0 (acid) to 14 (basic). A pH of 7 is neutral.
REFERENCES:
Bravo, H. R. Soto, R. Sosa, P. Sánchez, A. L. Alarcón, J. Kahl y J. Ruíz. Effect of acid rain on building material of the El Tajín archaeological zone in Veracruz, México. Environmental Pollution 144: 655-660. 2006.
Bouwman, A. F., D. P. Van Vuuren, R. G. Derwent y M. Posch. A global analysis of acidification and eutrophication of terrestrial ecosystems. Water, Air, and Soil Pollution 141: 349–382. 2002.
Calva, V. G., V. C. Flores., R. German., L. V. Ruz, R. M. Sánchez., T. A. Soto y R. Vázquez. Un fenómeno degradatorio de los bosques del Valle de México, la lluvia ácida. Revista Internacional de Contaminación Ambiental 7: 105. 1991.
EPA. Acid rain. 2011. Disponible en: www.epa.gov/acidrain/index.html. Fecha de consulta: abril de 2012.
Graveland, J. Effects of acid rain on bird populations. Environmental Review 6: 41-54. 1998.
INE. Aire. 2007. Disponible en: www.ine.gob.mx/ueajei/publicaciones/libros/16/parte4_17.html. Fecha de consulta: abril de 2012.
Muñoz, R., G. S. López-Venegas y A. Campos-Díaz. Estado de la lluvia ácida en la zona metropolitana del Valle de México. 2008. Disponible en: www.sma.df.gob.mx/. Fecha de consulta: abril de 2012.
Saavedra-Romero, D. Alvarado-Rosales, J. Vargas-Hernández y T. Hernández-Tejeda. Análisis de la precipitación pluvial en bosques de Abies religiosa, en el sur de la Ciudad de México. Agrociencia 37: 57-64. 2003.
SMAGDF, SMAGEM, Semarnat y Salud. Programa para mejorar la calidad del aire de la Zona Metropolitana del Valle de México 2011-2020. México. 2011.
Velasco-Saldaña. H. E., E. Segovia-Estrada, M. Hidalgo-Navarro, S. Ramírez-Vallejo, H. García-Romero, I. Romero, A. M. Maldonado, F. Ángeles, A. Retama, A. Campos, J. Montaño y A. Wellens. Lluvia ácida en los bosques del poniente del Valle de México. XXVIII Congreso Internacional de Ingeniería Sanitaria y Ambiental. 2002.
Xu, R. K. y G. L. Ji. Effects of H2SO4 and HNO3 on soil acidification and aluminum speciation in variable and constant charge soils. Water, Air, and Soil Pollution 129: 33–43. 2001.
|