| CHAPTER 5. ATMOSPHERE |
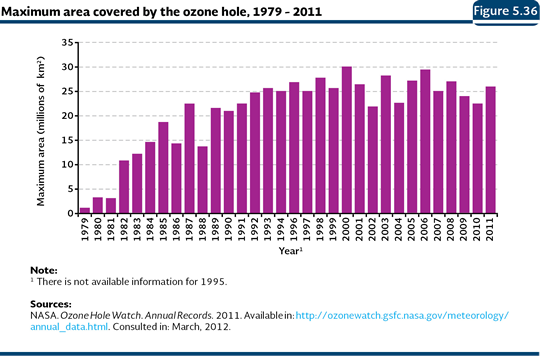
STRATOSPHERIC OZONE The thinning of the ozone layer is another of the global environment problems, because such layer regulates the ultraviolet light21 (UV) emitted by the sun. The destruction of the ozone layer has been the result of the action of several agents, generically known as ozone-depleting substances (ODS), which may exist naturally in the atmosphere or be generated as the result of the human activities; they have the distinctive characteristic to contain, in their structure, atoms of chlorine, fluoride and bromine. The most known anthropogenic ODS are the chlorofluorocarbons (CFC), but the hydro chlorofluorocarbons (HCFC), halons, methyl bromide (MBR), carbon tetrachloride (TET) and methyl chloroform are also important. The ODS are generally used in the systems for refrigeration, air conditioning, rigid polyurethane foam, solvents, pesticides, aerosols and extinguishers, among others. As they are emitted, the ODS reach the stratosphere where they take part of a series of reactions which free atoms of chloride and bromide that destroy the ozone molecule. To give an idea of the destruction capacity of these substances, a chloride or bromide atom may destroy up to a hundred thousand ozone molecules (WMO and UNEP, 2003). Although the ODS emissions are generated in the whole planet, and the thinning of the ozone layer happens at a global level, the atmospheric circulation moves most of the ODS towards the poles. In particular, the atmospheric conditions in the South Pole favor the reactions that turn the ODS into radioactive gases that destroy the ozone. During such reactions, chloride and bromide are released in active form that is accumulated in the polar clouds. In the spring, when the temperature raises, the clouds break up releasing active chloride and bromide, which rapidly destroy the ozone. Due to this, although the problem is global, its effects are minor near the Ecuador and they increase with the latitude towards the poles, in particular towards the South Pole (PNUMA, 2002, 2003; WMO and UNEP, 2003; Manney et al., 2011). The thinning of the ozone layer in the Antarctic has caused what it is known as the “ozone hole22”, observed for the first time at the beginning of the decade of 1980 and which showed its maximum recorded size in 2000, covering nearly 29.9 million square kilometers (Figure 5.36). In 2011, the maximum size was 26 million square kilometers, a surface slightly bigger than North America (NASA, 2011). Although the ozone hole had just exclusively been seen in the Antarctic, in 2011, it was recorded for the first time in the Arctic. That year, the cold conditions in the low arctic stratosphere lasted more than usual and they were more severe, which allowed the release of active forms of chloride that destroyed around 80% of the ozone molecules between 18 and 20 kilometers of altitude (Manney et al., 2011).
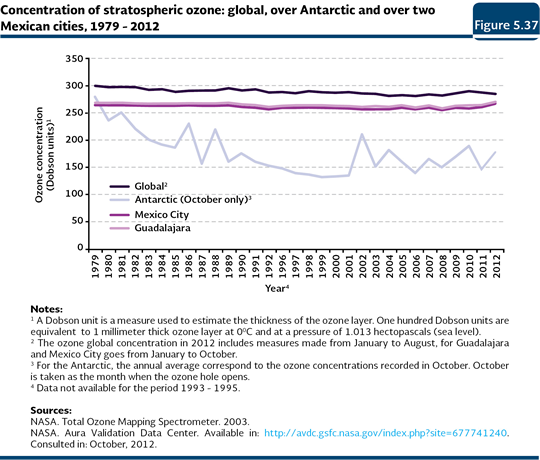
The North American Space and Aeronautics Agency (NASA) monitors the ozone concentration in the principal cities of the world, as well as in the poles. Figure 5.37 shows the annual average concentrations of global stratospheric ozone, in Mexico City, Guadalajara and Antarctic (in October23), between 1979 and 2012. It is evident the low concentration recorded since the beginning of the 1980s in October in Antarctic, compared to the global concentration (IB 1.3-3). In that region, there has been a steady decreasing tendency in the ozone concentration and although in some years, there have been increases, these have always been below the global concentration. In contrast, both the global concentration and the one from the two Mexican cities shown as a reference, although the present changes in the concentration, they are not meaningful, what reinforces the fact that in spite of being a problem generated at a global level, its more evident consequences are regional.
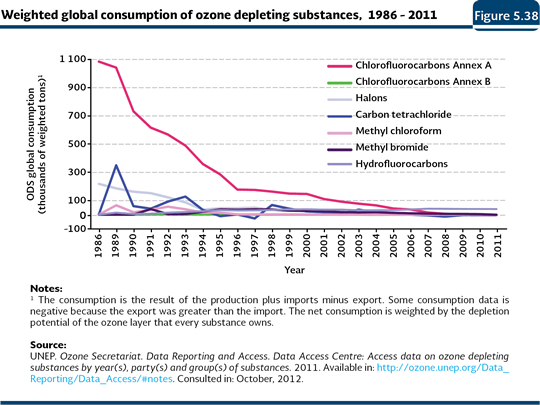
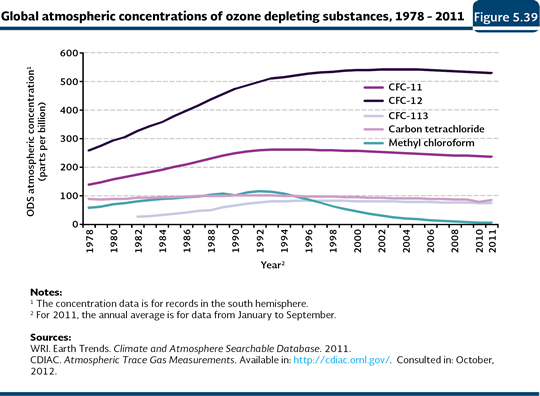
ODS consumption and concentration Since the impact of the ODS depends on their ozone depletion potential24 (WMO and UNEP, 2003), in this chapter, the global and national consumption, weighted by such potential, are presented. It is important to note that the weighted consumption of ODS considers, in an integral way, the life cycle of these substances (production, import and export), as well as their specific capacity to destroy the ozone. Although the ODS global consumption decreased dramatically in the beginning of the 1990s with the implementation of the Montreal Protocol25 (97% in the period 1986-2011), this has not been shown in their atmospheric concentration (Figures 5.38 and 5.39; IB 1.3-1 and 1.3-4). Although it would be expected their concentrations reduced almost at the same time as the consumption fall, they have settled down since the beginning of the 1990s, what may be explained by the times of the atmospheric life of the ODS, which may be long, going from five to 100 years (WMO and UNEP, 2003; CDIAC, 2011).
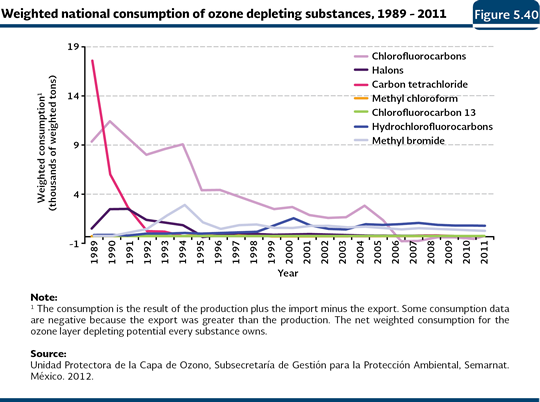
In Mexico, the total weighted consumption of ODS decreased about 95% in 2011 (1,563 tonnes were consumed) with respect to the reported volume in 1989 (slightly more than 29 thousand tonnes; Figure 5.40; IB 1.3-2; IC8). This decrease is caused mainly by the elimination of the consumption of the CFC with a higher depletion potential and by the increase in the use of alternative substances such as the HCFC with low depletion potentials.
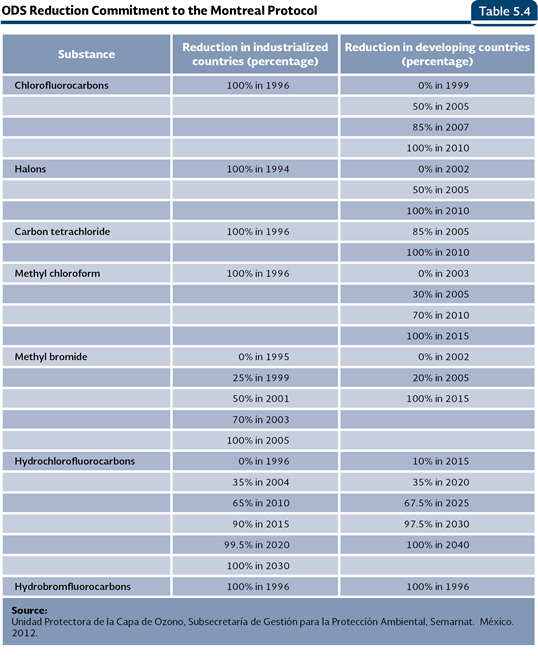
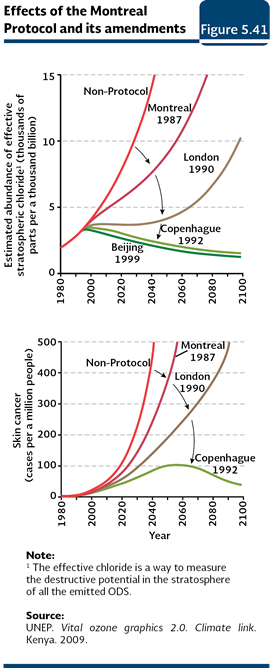
Besides the atmospheric concentration of ODS, there are other factors that influence the destruction of the ozone: the temperature in the stratosphere, the solar activity and the atmospheric concentration of gases such as the methane, water steam and the nitrous oxide (Weatherhead and Andersen, 2006). Notwithstanding the efforts made and the reached objectives in the reduction of consumption and production of ODS, the recent evaluations show the ozone layer will go back to the levels it had in 1980 between 2050 and 2075 (PNUMA, 2007 y 2008). Protection of the ozone layer The concern of the scientific community and the governments of several countries for the loss of the stratospheric ozone led to the adoption of the Vienna Convention for the Protection of the Ozone Layer (1995) and the Montreal Protocol on Substances that Deplete the Ozone Layer (1987), which established the commitments to reduce the consumption and protection of ODS (PNUMA, 2003; Table 5.4). By June 2012, 197 countries had signed and ratified both the Convention and the Protocol. Mexico signed these treaties and adopted the amendments of London (1991), Copenhagen (1994), Montreal (2006) and Beijing (2007; UNEP, 2011b).
Since 1995, most of the ozone depleting substances included in the Montreal Protocol, with exception of the CFC, had been stopped their production in the industrialized countries. From 2005, the global production and consumption of over 95% of the substances controlled by the Protocol was eliminated. In the case of the developing countries, the Protocol specified, in addition to a grace period for their elimination, financial support which would allow them to face the costs to eliminate the ODS.
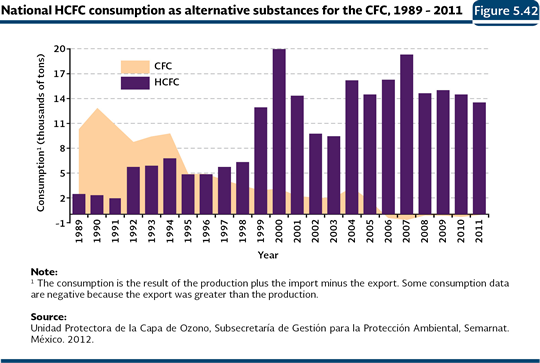
Mexico, just like other developing countries, committed to fulfill the ODS reduction goals shown in the Table 5.4. The reduction strategy followed by the country has been based on the following measures: 1) control of ODS consumption and production, 2) promotion and counseling about the use of substances and alternative practices that minimize the impacts in the ozone layer, 3) introduction of clean technologies that use ODS alternative substances and practices, and 4) training of the users about the measures to conserve the ozone layer. Such strategy is framed in the reduction calendar committed by the countries before the Montreal Protocol. Out of the goals committed by Mexico, the ones established for the CFC were met before the time limit. These were the carbon tetrachloride (TET), the methyl chloroform (MCF) and the halons. In September 2005, our country closed down our unique CFC production plant, with which the production in Latin America was reduced 60% and 12% at a global level, doing it four years before the established commitment fulfillment. Currently, in the country, all of the products in aerosol, refrigerators and air conditioners, as well as the production of polyurethane foams, are CFC free (Semarnat, 2009). From 2006, it was reported a CFC negative consumption, since the production of such substances was eliminated and part of the reserves was exported (Figure 5.40). Concerning the Methyl bromide, used as pesticide for the fumigation of farming lands and storing systems of crops and flours, the decrease has been progressive: in 2005, the goal to reduce 20% from the base line established in 1998 (1,130 weighted tonnes) was achieved, and between 2008 and 2011, 370 additional weighted tonnes were eliminated, so the total reduction of consumption of this substance in Mexico reached 53%. An important part of the reduction in the consumption of this substance has been done with the use of the graft technique in the vegetation production, what avoids some of their diseases and reduces their use as pesticides. Likewise, Mexico reschedules the total elimination of this substance one year before the established time, so it must be done by 2014. For more detailed information about production, import, export and consumption of ODS in Mexico, it is recommended to consult the Tables D3_AIRE03_01, D3_AIRE03_02, D3_AIRE03_03, D3_AIRE03_04 and D3_AIRE03_05. As part of the promotion to use ODS alternative substances, some substances which are less harmful to the ozone layer have been used. For instance, the HCFC owns a lower depletion potential: the ones used in Mexico own potential between 0.04 and 0.07, contrasts against the ones reported for the CFC, which go from 0.6 to 1.0. Figure 5.42 shows the results of the CFC substitution processes: while the CFC consumption was totally eliminated, as a result of the support of investment project on clean technologies in sectors that use these substances, the HCFC consumption has been increasing (IB 1.3-5). It is important to note that, even for the HCFC there are progressive goals of reduction in its consumption; in 2015 it must decrease 15% until it reaches 100% in 2040 (Table 5.4).
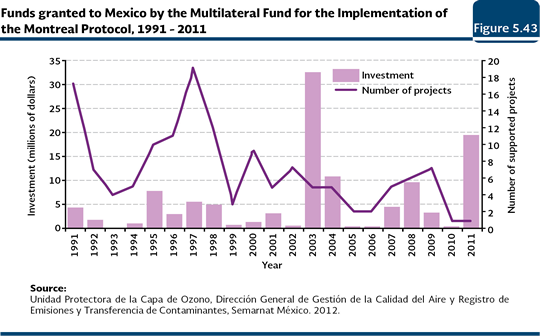
The Multilateral Fund for the Implementation of the Montreal Protocol (UNEP, 2011a) was established in 1991 to help the developing countries to fulfill the control measures adopted before the Montreal Protocol. The resources of this Fund are addressed to promote the introduction of clean technologies and the training of the ODS users. As of November 2011, this Fund had assigned about 2,800 million dollars at a world level to support 145 developing countries in the execution of 6,875 projects. The investment has allowed a reduction in the consumption and production; for instance, as December 2010, the consumption of 256, 180 tonnes was avoided as well as the production of 192,628 tonnes, in other words, 448,808 weighted tonnes of ODS (UNEP, 2011a). Mexico received from this Fund in the 1991-2011 period a total amount of 113.3 million dollars to support 143 projects in sectors such as refrigeration, aerosols, foams, solvents, agriculture and storing of products, among others (Figure 5.43).
Notes: 21 The ultraviolet radiation (UV) is a way of radiant energy that comes from the Sun and it reaches the Earth in the form of UV-A, UV-B and UV-C. UV-A light is the least harmful and they come to the earth surface in a small quantity. UV-C rays are the most harmful, due to their high energy content, but the ozone layer prevents them from going through the atmosphere. Finally, the UV-B, it is also very harmful, it is mostly retained by the ozone layer, although a little part of it reaches the surface and it may cause damage to the cells and tissues of the organisms.
|